Yeast Stress & Viability
Yeast Stress & Viability
Yeast stress is a vast topic that is nowhere near being fully understood, however, to begin scientists have come up with different ways to measure it. The term most used for the measurement of a culture’s health is viability. Viability is the ratio of live yeast cells to dead yeast cells. This can be measured a few different ways, the most basic of
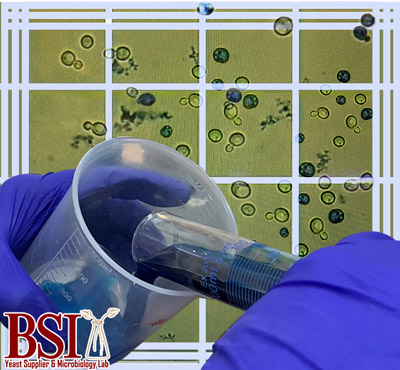
which is called methylene blue staining (Smart, Chambers, Lambert, Jenkins, & Smart, 1999). In addition to methylene blue, there are also more accurate molecular methods for measuring viability with the use of stains like propidium iodide and acridine orange. To get the true picture of the health of a culture, vitality also needs to be measured. Vitality is the measurement of the metabolic activity of yeast. The current methods for measuring this are highly varied and are not incredibly accurate. Methods range from testing glycogen content to measuring esterase activity (Chan, Driscoll, Kuksin, & Saldi, 2016). Due to the ever-changing nature of these, measuring vitality is like hitting a moving target.
During the brewing process yeast is exposed to an environment that is not always ideal for its health. To manage these conditions, cells undergo considerable physiological changes. Among many other things, cells can alter their cellular membrane to handle adverse conditions by increasing sterol content, they can increase internal glycerol concentrations to deal with osmotic stress, and they can build proteins that help them tolerate temperature fluctuations. In a brewery the main environmental stressors are temperature, pressure, osmolarity, oxidative stress, pH, and nutrient depletion. Time exposure to each of these plays an important role in the severity of viability loss.
Baseline industry standards for these vary widely, but here are a few basic guidelines to help improve the longevity of your yeast.
• Temperature:
o After reaching terminal gravity harvest yeast as soon as possible and store at 34F.
o During fermentation be sure to adhere to strain specific temperature requirements.
• Pressure
o After reaching terminal gravity harvest yeast as soon as possible to limit the amount of time yeast is exposed to hydrostatic pressure from the beer above.
o During yeast brink storage limit the amount of head pressure to between 0-2psi.
• Osmolarity
o Generally brewing above 10 Plato increases the quantity of genetic mutations that will occur. To prolong the life of your yeast, try to remain as close to 10 Plato as possible. If brewing over 10 Plato, it is not recommended to reuse any yeast from that brew.
• Oxidative stress
o Maintain a DO of around 8-10ppm. This will provide enough oxygen for yeast’s sterol synthesis.
o An inline knockout oxygenation rate of between .5-1LPM will get you close to 8-10ppm, however, several factors like wort temperature and gravity can affect how much oxygen stays in solution. Careful considerations need to be made when dealing with these conditions.
• pH
o A starting fermentation pH of around 5.2-5.4 and an ending pH of 4.2-4.4 will help maintain yeast viability.
o When brewing sour beers serial re-pitching is not advised.
o Harvest yeast once terminal gravity has been reached so that carbonic acid build-up does not adversely affect viability.
• Nutrient depletion
o Along with macronutrients like carbohydrates and fatty acids, yeast also need a supply of micronutrients like zinc and calcium. However, be sure not to oversupply the yeast with micronutrients as this can also have a detrimental effect.
o An adequate supply of free amino nitrogen (FAN) is also required for yeast to build essential proteins.
If these base requirements are continually met, yeast can theoretically be re-pitched indefinitely. However, as we all know, the brewhouse cannot always meet these requirements due to product demands from the customer. If your yeast begins to decline in viability over time it may be time for a fresh pitch. For any questions on how to order or on how to maintain yeast health, please contact our technical support team at, 719-482-4895 x 3.
By Kory Davis
QC & Propagation Specialist
The Brewing Science Institute
References
Smart, K. A., Chambers, K. M., Lambert, I., Jenkins, C., & Smart, C. A. (1999). Use of Methylene Violet Staining Procedures to Determine Yeast Viability and Vitality. Journal of the American Society of Brewing Chemists, 57(1), 18-23. doi:10.1094/asbcj-57-0018
Arroyo-López, F. N., Orlić, S., Querol, A., & Barrio, E. (2009). Effects of temperature, pH and sugar concentration on the growth parameters of Saccharomyces cerevisiae, S. kudriavzevii and their interspecific hybrid. International Journal of Food Microbiology, 131(2-3), 120-127. doi:10.1016/j.ijfoodmicro.2009.01.035
White, P., Kennedy, A., & Smart, K. (2008). The Osmotic Stress Response of Ale and Lager Brewing Yeast Strains. Brewing Yeast Fermentation Performance, 46-60. doi:10.1002/978047069604
Chan, L., Driscoll, D., Kuksin, D., & Saldi, S. (2016). Measuring Lager and Ale Yeast Viability and Vitality Using Fluorescence-Based Image Cytometry. Technical Quarterly. doi:10.1094/tq-53-1-0216-01
Palou, E., López-Malo, A., Barbosa-Cánovas, G. V., Welti-Chanes, J., Davidson, P. M., & Swanson, B. G. (1998). High Hydrostatic Pressure Come-Up Time and Yeast Viability. Journal of Food Protection, 61(12), 1657-1660. doi:10.4315/0362-028x-61.12.1657
Fernandes, P. M. (2008). Saccharomyces cerevisiae Response to High Hydrostatic Pressure. High-Pressure Microbiology, 145-166. doi:10.1128/9781555815646.ch8

